With the Stryker Accolade V40 cases, we are looking for two different types of injuries:
1. Metallosis cases – These cases resemble other metal-on-metal (MoM) and modular hip implant cases, where patients present with increased levels of cobalt and/or chromium, pseudotumors, bone and tissue damage, inflammatory fluid around the implant, development of severe pain several years after the index surgery, need for revision surgery due to premature device failure, etc.
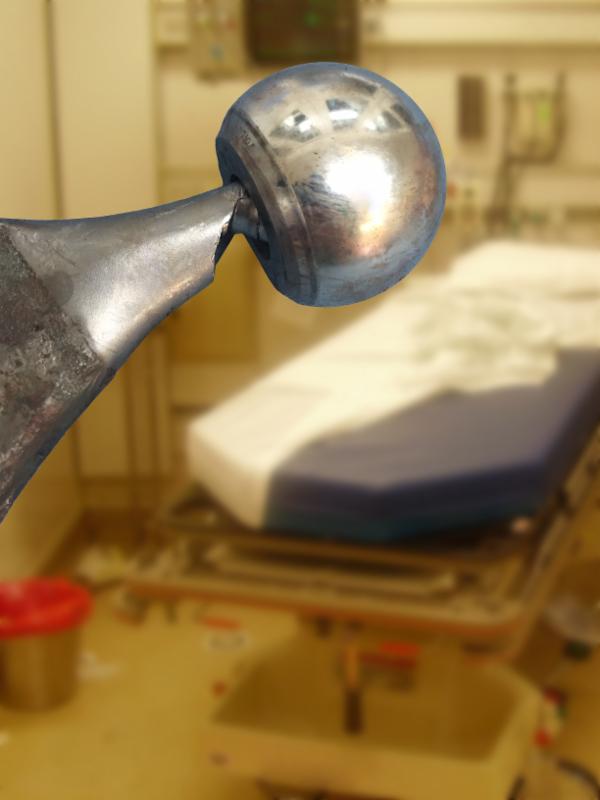
2. Dissociation cases – These cases are rarer, but occur when the V40 taper is ground down by the metal head into a bird beak shape. This corrosive process is particularly violent, and the patient will present with a dissociated head. In other words, the actual head of the implant will fall off and separate from the neck of the implant, which causes catastrophic injuries to the patient and often occurs without any warning.